ABOUT ATLAS LINK
Atlas Link Technology Group, founded in 2004, specialises in the design, development, manufacturing, and distribution of IVD reagents and instruments, and provides a range of advanced services to the global IVD industry. The company's R&D and commercial headquarters are located in Beijing, China, with additional independent sales offices in Virginia (USA). Over the past two decades, Atlas Link Technology Group has grown into a global high-tech enterprise with more than 200 employees. In recent years, the company has intensified its focus on advanced technology development and has secured multiple international patents. Its service portfolio has expanded to include co-development partnerships and regulatory support. The R&D centre is equipped with state-of-the-art capabilities and offers third-party testing services. Atlas Link Technology Group owns several internationally recognised trademarks, including NOVAtest and Sensotest.
The company's main manufacturing site is located in Langfang, Hebei Province, China, and serves as Atlas Link Technology Group's largest production base for rapid IVD test kits. Covering a total area of 23,000 m², the facility features fully automated and semi-automated production lines.
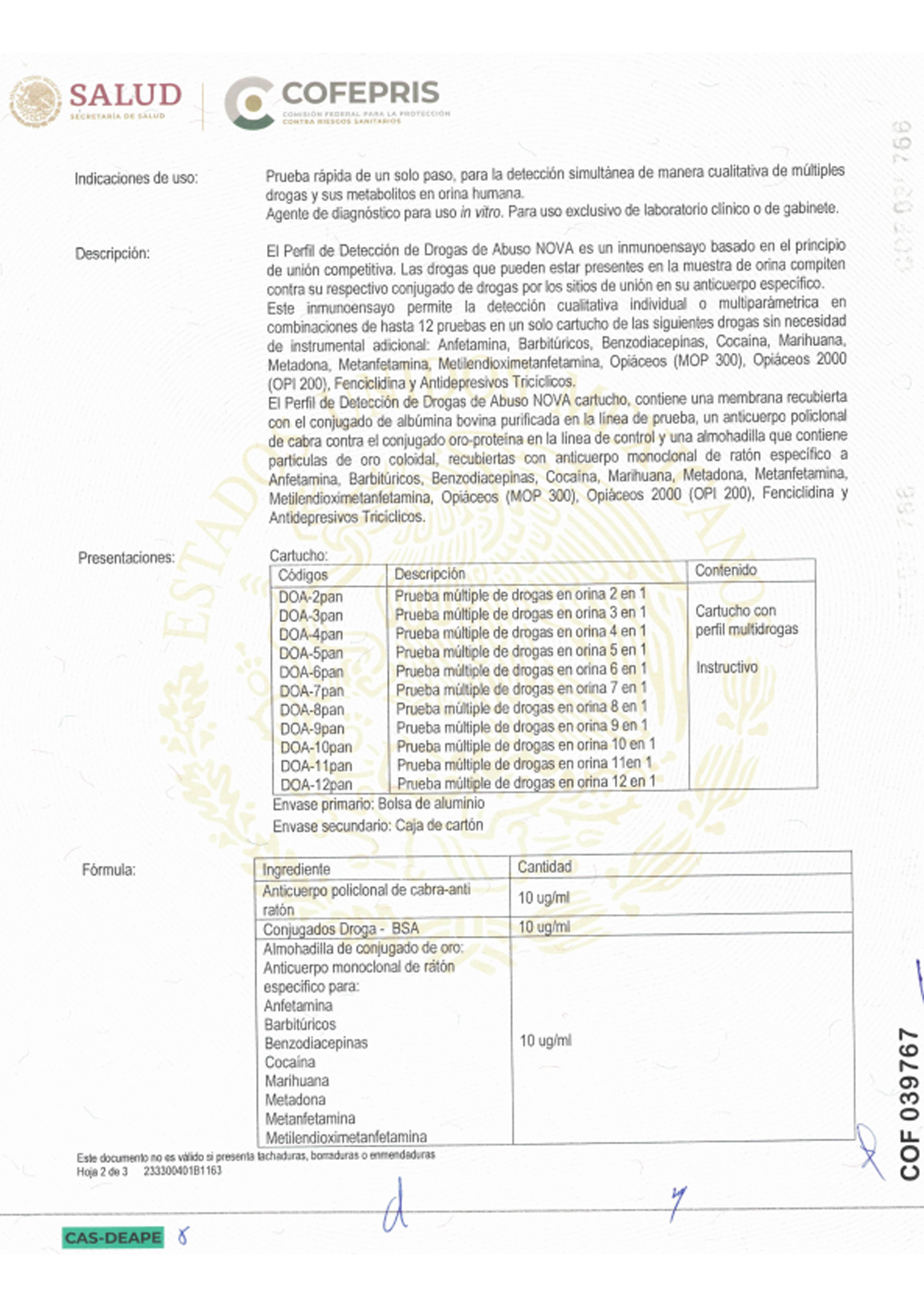
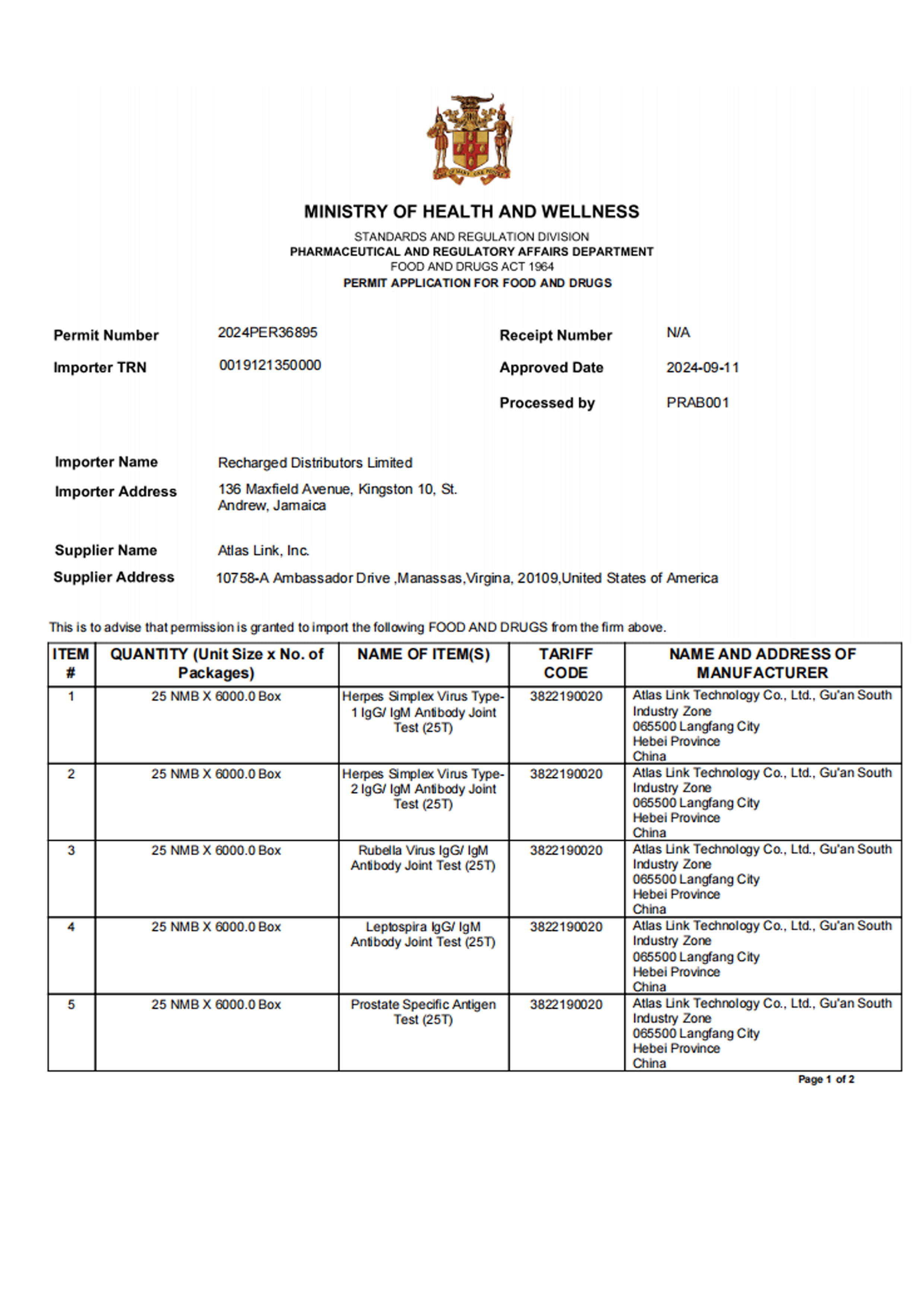
Atlas Link Technology Group offers a portfolio of more than 200 products across multiple diagnostic platforms, including colloidal gold immunochromatography, immunofluorescence, ELISA, dry chemistry, and molecular diagnostics. Its test menu covers fertility assessment, infectious diseases, vector-borne diseases, sexually transmitted infections, markers, clinical indicators, and drugs of abuse, supporting both professional use in laboratories and POCT settings, as well as home self-testing. The company has obtained EU CE certifications (Including IVDR), U.S. FDA 510(k) approval, China NMPA certifications, and product approvals across Southeast Asia, South America, Africa, and other regions.
BETA-BEAR LABORATORY

Beta-Bear Advanced Standardization Lab is a wholly funded research unit established by Atlas Link Technology Group to support the precise development of in vitro diagnostic reagents. The laboratory is a core component of Atlas Link Technology Group’s "R&D–Sales–Production" strategic framework and represents the most revolutionary organisational innovation the company has introduced in the past two decades.
The Lab is equipped with advanced research instruments from leading brands such as Thermo-Fisher and Eppendorf, including Class II Biological Safety Cabinets (BSC), clean benches, microplate readers, ultra-low temperature storage systems, micro-volume UV-Vis spectrophotometers, and centrifuges, positioning it at the forefront of technological capability in the industry. The current team consists of more than ten technicians, led by an R&D supervisor with over ten years of experience. Core team members come from top 50 universities in China and around the world. As of 2025, the Lab has independently developed more than 160 new products, demonstrating strong in-house innovation capabilities.
In addition to its research and development strengths, the Lab has also established a small internal GMP production workshop dedicated to the customised development and manufacturing of products.
GLOBAL REGISTRATION TEAM
Our Registration Support Team is composed of experienced professionals with diverse backgrounds in law, computer science, and biology. Most team members have been with our company for more than three years, giving them an in-depth understanding of our products and technical documentation requirements. The team has successfully secured registrations for major markets, including CE IVDR, FDA 510(k), China Class II medical devices, as well as approvals in Saudi Arabia, South Korea, Brazil, Colombia, Indonesia, Romania, and many other countries.
We provide comprehensive registration services tailored to customer needs. This includes preparation of registration dossiers, technical documentation, clinical or performance evaluation guidance, and submission-ready documents. For clients who only require documentation packages, we deliver complete, audit-ready materials that comply with relevant local regulations.
Driven by accuracy and reliability, our Registration Support Team ensures that every submission meets regulatory requirements, helping our partners achieve timely and compliant market entry worldwide.

COMPLIANCE&CERTIFICATE
IVDR-CE QMS
MISSION, VISION & VALUES
MISSION
To empower global health with precise, accessible, and reliable diagnostic solutions.
We innovate and manufacture high-quality IVD reagents and instruments, advancing clinical insight and improving health outcomes for laboratories, healthcare providers, and families worldwide.
VISION
To become a world-leading biotechnology group that sets the benchmark for rapid diagnostics, scientific innovation, and global trust.
We envision a future where everyone—regardless of geography—can benefit from accurate, timely, and affordable diagnostic technologies.
Through continuous innovation, global collaboration, and a commitment to excellence, we aim to elevate the standards of the IVD industry.
VALUES
Innovation With Purpose
We push the boundaries of scientific discovery to deliver meaningful diagnostic solutions that improve lives.Quality Without Compromise
Quality Without Compromise
Every product reflects our dedication to precision, safety, and global regulatory excellence.
Integrity & Responsibility
We operate with transparency, respect, and accountability—because trust is the foundation of healthcare.
Global Collaboration
With teams and partners across continents, we embrace diversity and share knowledge to create greater impact.
Customer-Centric Excellence
We listen, support, and adapt—ensuring that every user, from clinicians to families, receives dependable and intuitive diagnostic tools.
Sustainability & Long-Term Development
We are committed to responsible operations, sustainable manufacturing, and contributing to the future of global public health.